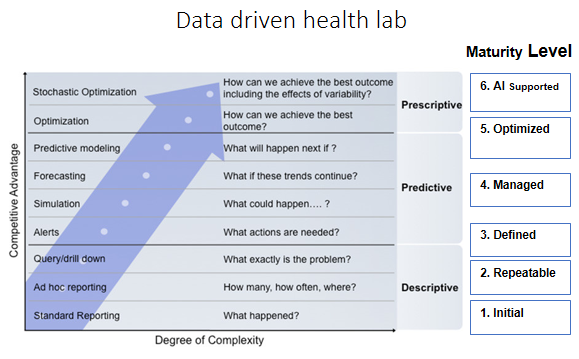
Data driven Health Lab vision
back to Data driven health lab Call to Action
 Digitization of diagnostic health services has begun at the Radiology and Clinical Chemical Labs. Clinical Genetics and Pathology Labs follow this trend at a high pace. The digitization creates a much clearer difference between doing the test/experiment and its analysis / interpretation including making a report with the conclusions and advice for follow-up. Because the lab/medical device is no longer tested in the same environment, it is necessary to separate these services from the analysis and the report. For each lab information and technology will be a key resource. For top institute labs that need to innovate especially data/information becomes a strategic asset when it's used to differentiate and create more patient, research, education and valorization value. The organization culture needs to change to become a smart AI supported data driven health lab. From experimental internal focused to a co-operative mindset that is part of a network that wants to share and continuously improve.
Digitization of diagnostic health services has begun at the Radiology and Clinical Chemical Labs. Clinical Genetics and Pathology Labs follow this trend at a high pace. The digitization creates a much clearer difference between doing the test/experiment and its analysis / interpretation including making a report with the conclusions and advice for follow-up. Because the lab/medical device is no longer tested in the same environment, it is necessary to separate these services from the analysis and the report. For each lab information and technology will be a key resource. For top institute labs that need to innovate especially data/information becomes a strategic asset when it's used to differentiate and create more patient, research, education and valorization value. The organization culture needs to change to become a smart AI supported data driven health lab. From experimental internal focused to a co-operative mindset that is part of a network that wants to share and continuously improve.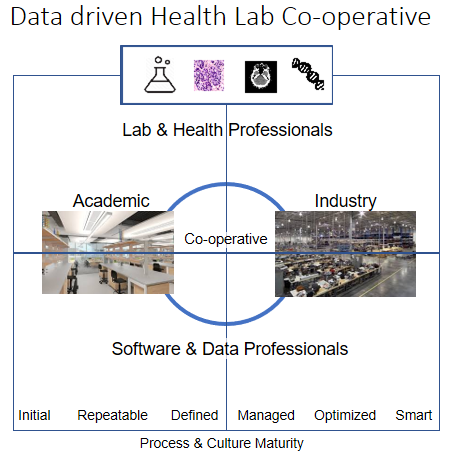 The data driven Health Lab Co-Operative figure shows the challenge of connecting the Academic Lab world with the Industry world. For both worlds the Lab & Health professional communities need to be supported by Software & Data Professional communities. The vacuum between these communities and worlds is the center for new value creation by entrepreneurs. It requires a co-operative sharing approach that develops into a data driven health ecosystem driven by the combination of:
The data driven Health Lab Co-Operative figure shows the challenge of connecting the Academic Lab world with the Industry world. For both worlds the Lab & Health professional communities need to be supported by Software & Data Professional communities. The vacuum between these communities and worlds is the center for new value creation by entrepreneurs. It requires a co-operative sharing approach that develops into a data driven health ecosystem driven by the combination of:
- Cloud/mobile technology platforms
- Scarce top Lab & Health professional expertise commitment
- Scarce top Software & Data professional expertise commitment
- Citizen 'free flow of health' data empowerment
- Safeguarding legal issues regarding Privacy and Data protection as well as Intellectual Property.
The co-operative space is a triple helix of public, private and citizen participatives also called ‘commons’. All of these parties have to ensure that the health data is used in a trusted and FAIR way for their citizens.
The market opportunity is described by McKinsey: Why evolving healthcare services and technology market matters
The US EBITDA 2021 market is $50 billion with the strongest CAGR >10% for software platforms and technology and data analytics and information services.
In Europe the Digital Health Society initiative of 100 million Digitally Connected Healthy EU Citizens by 2027 has been started.
|
|
Population |
Genetic Tests |
|
Estonia |
1.315.600 |
100.000 |
|
Netherlands |
17.081.500 |
1.298.381 |
|
Germany |
82.800.000 |
6.293.706 |
|
Rest of Europe |
410.608.000 |
31.210.702 |
|
US |
325.700.000 |
24.756.765 |
|
Asia-China |
1.411.000.000 |
107.251.444 |
The Global Screening Array Consortium is a great opportunity to introduce a cost effective test for a genetic citizen passport. Estonia has started for 100.000 of their citizens and ErasmusMC already has 1.100.000 citizens via their GSA research lab co-operative. The co-operative manages a >25 million reference collection. The table shows the potential if this test is rolled on a European, US and Asian/Chinese scale.
Trends & background developments
- The data driven lab development is being intensified by the fact that patients and possibly research respondents will have the data portability right to get a copy of their (raw) data copy (blue button). They can use this right to search for the best quality/price for the performance of the test and the expert outcome. For a second opinion, this will certainly be done in an international knowledge/expertise network. In addition, the GDPR (European Privacy Act) enforces any institution that has privacy sensitive (clinical and research) data to demonstrate that they have a data sharing process as well as their information security in place and auditable.
- The standard tests will be purchased at the best price / performance that is determined by volume, including standard analysis. The analysis and diagnostic / interpretation support software can basically be delivered by any trusted cloud service. The challenge is to get the (standard) lab / instrument data safely (with privacy and security by design) to the cloud and give users secure access to this cloud for their analysis.
- All service components of lab, analysis and diagnostic interpretation are no longer combined in one department / institute but consist of a network of services. This necessitates an orchestration/chain service, in which experts work together on the integral process quality and the associated costs. The process and the associated data of application (box), to DNA material logistics, to lab should be available for audits.
- Care and research data will be available for all stakeholders through Trusted Digital Repositories from a shared responsibility (as curator). This means that lab data collections will be available for the (diagnostic) department together with their clinical and research clients and on individual data level for the patient/respondent/citizen for privacy by design audits and personal requests!
- The International Health Data Standards Organization has recently published its 1st publication for clinical sequencing labs. This is a sign that the international market has gone to the next stage of digitization and standardization
Data driven EU Health Lab Shared Service
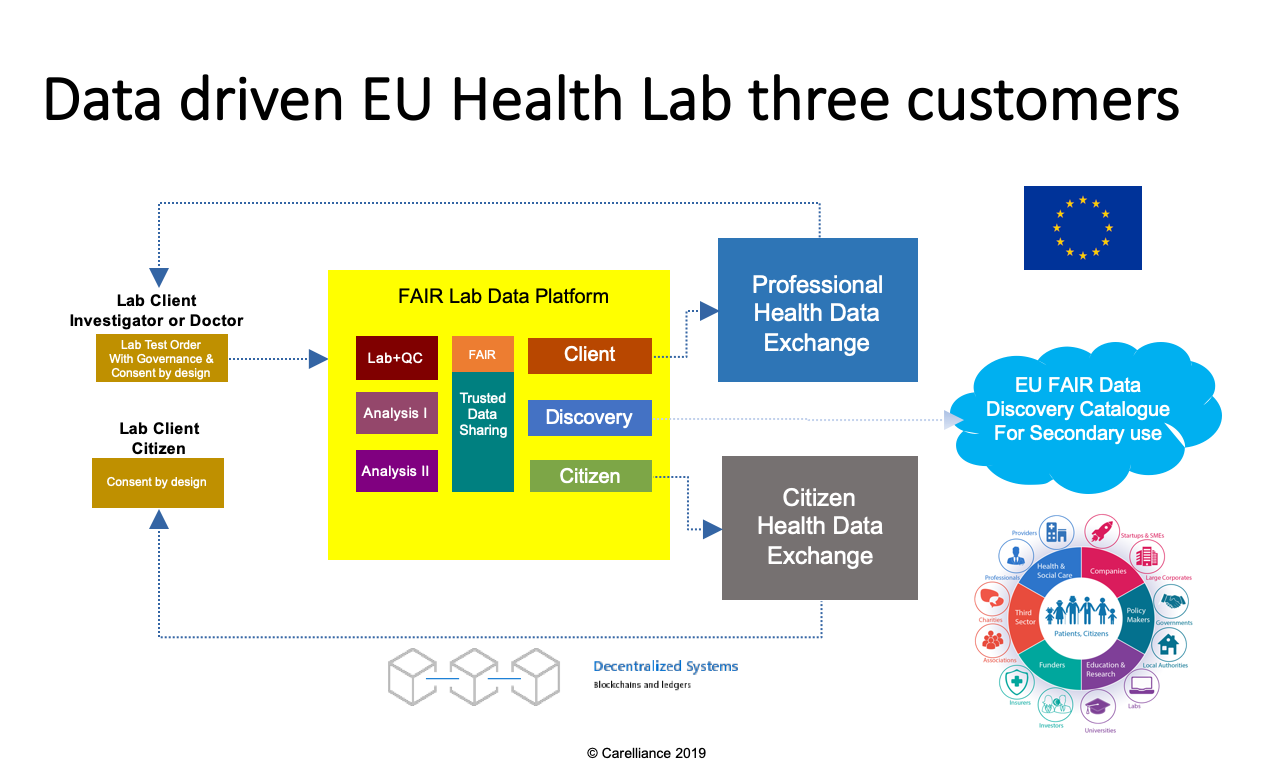
This figure describes the data driven EU lab process and data service links. The data service is designed from three Lab Customer/Client perspectives.
- Client order process.
- The lab runs both services for clinicians (Doctors) and researchers (Principal Investigators).
- Because of the GDPR (Act 20 Data portability) the EU citizen becomes a Lab data service client.
- The data is available for discovery in a European FAIR Catalogue. The citizens consent determines the secondary use of this data.
- The order process is standardized using IHE interoperability standards.
- Shared Lab Data platform with the following services
- Lab instrument and Quality Control (QC). Lab instrument(s) wet process to turn biomaterial samples into data. Output data = (Raw) instrument file(s).
- Analysis I. First level (Instrument) Analysis. Mostly linked to instrument supplier and QC process. Output data = Processed instrument file(s). + Analysis I Report
- Analysis II. Second level Analysis that is used to support diagnostic and discovery process. Output data = Processed Analysis I file(s) + Analysis II Report
- Digital Repository to archive data sets independent from instruments and applications.
- Trusted Data and Standard Operating Procedure Sharing with a FAIRdata collection (catalogue) publishing service component.
- Data sets are managed as archival packages for clients, citizens and discovery (catalogue) services.
- Health data exchange platforms
- For the health professionals, data is shared via Electronic Medical Records (EMR) and (Regional/National) Health Information Exchange (HIE) platforms. Data is available as download archival package or as linked data set to a trusted (Analysis I or II) processing service.
- For the citizens, data is shared via Personal Health Records (PHR) and Citizen Community Safe services. Data is available as download archival package or as Trusted Linked Data set to a trusted (Analysis I or II) processing service.
- For secondary EU Digital Single Market (DSM) discovery use. Data is available as a FAIR catalogue and is linked to the (Trusted) Digital Repository services.
- The data interoperability levels are well described by Tim Berner Lee in his great Linked Data article.
- Health data Exchange transport technologies based on standards
- All are TCP-IP (internet) based transport
- (Secure) http(s)/(s)ftp file or API/REST based
- With interoperable metadata for Health Information Exchange services
- XML based (XDS/XDR)
- JSON based (FHIR)
- Decentralized IDentity supported by blockchain technology for personal citizen and health professonial access
- Depending on the service:
- Metadata sharing for registries and catalogues
- Archival packages sharing for data sets
- Trusted links sharing processed data sets
- Processing algorithms to process data sets
- All are TCP-IP (internet) based transport
- Free flow of Health Data in the European Digital Single Market
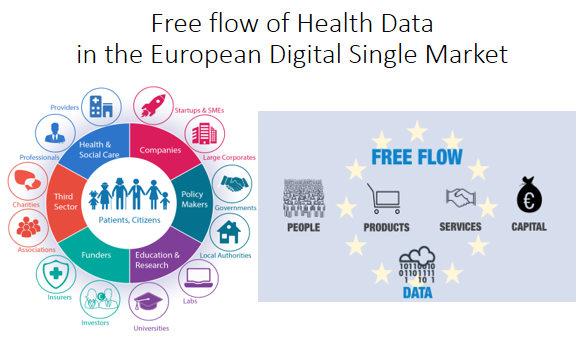
This figure shows the EU ambition to create a Digital Single Market with free flow of health data for their citizens. The Shared Lab Data platform is part of the EU Health Data Market for trusted data and applications. It offers it's services directly on the EU end-user citizen, researcher and health professional level. The GDPR will drive the harmonization process. Data driven lab leaders that comply have a competitive advantage, because they can directly offer their services on a European market level.
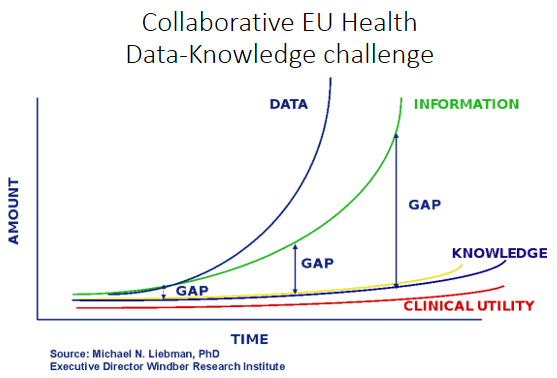
This figure shows the data, information, knowledge to clinical utility challenge. The clinical utility results in short and long-term health value for the citizen. The gap between data and utility needs to be closed by big data co-operatives on a international United Nation level. On EU level a 'coalition of the doing' is started. Inspired by Obama’s US moonshot and the following Chinese initiative, the EU wants to collect all health data of 100 million European citizens to support big data research and innovation.
The question for each top investigator is:
- Which breakthrough research could you achieve using this EU-US-Chinese collection?
- Which data collection and knowledge can you with your expert network add to this collection?
The term investigator should be interpreted very broadly. It’s not limited to (scientific) research but applies to any person who can add to the discovery value.
Download the paper here.
